Abstract
In myeloid malignancies, presence of 'multi-hit' TP53 mutations is associated with lack of response to conventional therapy and dismal outcomes, particularly when found in combination with a complex karyotype. Therefore, it is crucial to understand the biological basis of TP53-mutant driven clonal evolution, suppression of antecedent clones and eventual disease transformation to inform the development of more effective therapies. Myeloproliferative neoplasms (MPN) represent an ideal tractable disease model to study this process, as progression to secondary acute myeloid leukemia (sAML) frequently occurs through the acquisition of TP53 missense mutations.
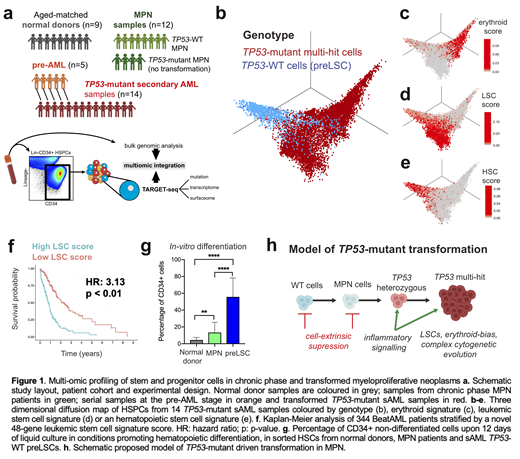
To characterize tumor phylogenies, cellular hierarchies and molecular features of TP53-driven transformation, we performed single-cell multi-omic TARGET-seq analysis (PMID: 33377019 & 30765193) of 22116 hematopoietic stem and progenitor cells (HSPCs) from 35 donors and 40 timepoints (controls, MPN in chronic phase, pre-AML and TP53-mutated sAML; Figure1a). TARGET-seq uniquely enables single-cell mutation analysis with allelic resolution with parallel transcriptomic and cell-surface proteomic readouts.
We invariably identified convergent clonal evolution leading to complete loss of TP53 wild-type alleles upon transformation, including parallel evolution of separate TP53 "multi-hit" subclones in the same patient (n=4/14) and JAK2-negative progression (n=2/14). Complex clonal evolution driven by chromosomal abnormalities (CAs) was present in all patients and TP53 multi-hit HSPCs without CAs were rarely observed. Subclones with recurrent CA such as monosomy 7 showed upregulation of RAS-associated transcription and preferentially expanded in xenograft models. Together, these data indicate that TP53 missense mutation, loss of TP53 wild-type allele and cytogenetic evolution are collectively required for leukemic stem cell (LSC) expansion.
Integrated transcriptomic analysis of sAML samples (Figure1b) revealed three major populations: (1) a TP53-mutant cluster (Figure1c) characterized by an erythroid signature (e.g. KLF1, GATA1, GYPA; an unexpected finding as no cases showed diagnostic features of erythroid leukemia), (2) an LSC TP53-mutant cluster (Figure1d) and (3) a TP53-WT preleukemic cluster (Figure1e). The LSC cluster showed dysregulation of key stem cell regulators, from which we derived a novel 48-gene LSC score with prognostic impact in an independent AML cohort (HR=3.13; Figure1f). Importantly, this score was predictive of outcome irrespective of TP53 status for both de novo and sAML, demonstrating its broader potential clinical utility.
TARGET-seq analysis uniquely allowed us to characterize rare TP53-WT preleukemic cells (preLSCs), which were almost exclusively confined to the immunophenotypic lineage-CD34+CD38-CD90+CD45RA- HSC compartment. PreLSC from sAML samples presented increased stemness, increased quiescence, aberrant inflammatory signaling and differentiation defects (Figure1g) as compared to HSCs from normal or MPN donors, both at the transcriptional and functional levels through in vitro long-term and short-term cultures. This indicates cell-extrinsic suppression of residual TP53-WT hematopoiesis.
Longitudinal analysis of TP53-heterozygous mutant HSPCs at different stages of disease evolution (Figure1a) revealed that aberrant inflammatory signalling (e.g. BST2, IFITM1, IFITM3) in the genetic ancestors of TP53 "multi-hit" LSCs, but not the presence of TP53-mutations alone, was predictive of subsequent transformation. In a mouse model system, TP53-mutant cells challenged with sustained inflammatory stimuli acquired a mean 3-fold competitive advantage in WT: TP53 R172H/+chimeras. This indicates that pro-inflammatory cues from the tumour microenvironment promote fitness advantage of TP53-mutant cells whilst supressing antecedent clones.
In summary, we present a comprehensive single-cell multi-omic analysis of the genetic, cellular and molecular landscape of TP53-mediated transformation, providing unique insights into the evolution of chronic hematological malignancies towards an aggressive acute leukemia (Figure1h). Since TP53 is the most commonly mutated gene in human cancer, we anticipate these findings will be of broader relevance to many other cancer types.
Kretzschmar: Vanadis Diagnostics, a PerkinElmer company.: Current Employment. Drummond: BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Harrison: Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sierra Oncology: Honoraria; Constellation Pharmaceuticals: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mead: Abbvie: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal